Jump to topic
Search
Principles of Health and Safety
The Penn State CHEER team recognizes that some communities have distrust in research. CHEER and the entire Penn State research community operate under strict federal guidelines that prioritize the safety, confidentiality, and proper treatment of human participants in research. All studies involving people are controlled under The United States Department of Health and Human Services (HHS) and the Office for Human Research Protections (OHRP). HHS and OHRP provide definitions and regulations for human research participants including protections for special populations in research.1
Did You Know?
Ethical protections and oversights to protect human subjects in research became regulated for the first time in 1948 through the Nuremberg Code when 23 Nazi physicians were tried for crimes against humanity during the Nuremberg Trials.30, 31 Additional ethical protections for human subjects participating in biomedical and behavioral research were created when the National Research Act was established in 1974, directly after the atrocities of the Tuskegee Syphilis Study. To learn more about the history of research protections, go to the University of Nevada, Las Vegas’s History of Research Ethics.32
In addition to following guidelines provided by HHS and OHRP, research teams are required to present study information to their organization’s Institutional Review Board (IRB), a department that oversees the protection of human participants in research. Penn State’s IRB is responsible for the review of all research involving human beings to reduce any potential harm or risk to “their physical and psychological well-being, confidentiality, [and] privacy”.27 Before any research can begin, an application and other required paperwork needs to be completed by the research team and submitted for the IRB to review and determine if the research can be safely and ethically conducted. Even if the researcher does not think there is any risk to the study participants, the IRB must review all parts of the study for consideration.
The HHS, OHRP, and the Penn State’s IRB ensure that all research studies involving human research participants hold to the following principles:
- Risks to research participants are minimized
- Risks are reasonable in relation to any anticipated benefits
- Selection of research participants is fair and equitable
- Protections are in place for vulnerable, at-risk populations
- Informed consent (written permission) will be sought; this consent will be documented or appropriately waived
- Plans will be put in place to ensure the safety of research participants.28
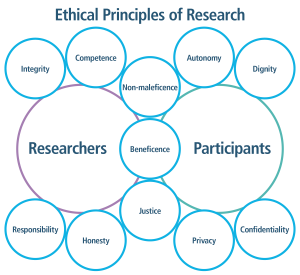
Participants’ health, safety, and confidentiality are priorities of research.29
Insider Tip!
Penn State offers detailed tutorials about topics related to human subjects research, including health and safety of study participants, through the CIRTification (Community Involvement in Research) program. For more information about this interactive, online training, go to Section 6: Training and Educational Offerings.
Principles of Engagement
Meaningful collaborations between community partners and researchers in CEnR are shaped by engagement principles that build success and benefit for everyone involved. CEnR partnerships value co-learning and are built on a foundation of trust, integrity, and mutual respect.33 Because underrepresented communities have historically been overlooked, deceived, and abused in academic and medical research, it is important that community partners taking part in CEnR are empowered by their experience as key stakeholders in the research project.34 Successful CEnR projects are guided by the following principles that result in benefits to those involved:
A True Partnership!
- Collaboration is the foundation of CEnR. Partnerships are a shared experience of creating knowledge that empowers all participants.
- Diversity in CEnR is more than the representation of specific groups. It is deliberately inclusive and welcoming of all participants and cultures.
- For CEnR to be successful, the process should benefit all stakeholders involved.
- Engagement is a process that happens throughout the life of the research project.
- Community partners are equally important to the research project as the researchers.
- Respect, compromise, flexibility and open communication are key skills for community partners and researchers involved in CEnR.
Community Priorities Matter!
- The interest(s) of the community is an important focus in CEnR.
- The community is viewed as an extension of the identity and culture of its members.
- Each community has a unique culture, history, set of concerns, hopes and resources that are as valuable to the research process as formal training and education.
- It is the responsibility of the research team to learn about the community with which they wish to collaborate.
Communication is a Two-Way Street!
- Trusting relationships between stakeholders is the result of transparent, honest
- The roles and boundaries of all stakeholders are communicated clearly and established early in the research process.
- Stakeholders should feel comfortable to share knowledge that contributes to the project and its outcomes.
- Technical terms are communicated by researchers in a way that is easily understood amongst community partners.
- Findings and knowledge gained by researchers are shared in an equal, timely manner to community partners.
- Regular evaluations of project progress (or lack thereof) should be discussed openly and communicated amongst all stakeholders.
- All participants are made aware of their rights to privacy and confidentiality.15,19,35,36
“Being a partner on the research project was an interesting, enjoyable and life-changing experience. The team helped us feel needed, engaged us in every project and always valued our opinions.”
– Patient Partner, PaTH to Health: Diabetes, A Patient-Centered PaTH to Addressing Diabetes: Impact of health policies on health outcomes and disparities
Ethical Principles of Research graphic. For researchers, integrity, competence, responsibility and honesty are listed. For participants, autonomy, dignity, privacy and confidentiality are listed. Non-maleficence, beneficence and justice are listed for both.
